Today I was doing some chromosome counts on a couple of seedlings and learned that one of the seedlings of the ‘Gaye Hammond’ rose is diploid! GH is tetraploid and likely an unfertilized egg developed into an embryo. When there are seedlings from crosses that don’t look like they have traits of their male parent and are more petite in size and more well branched than typical from tetraploids, I suspect that such seedlings may be diploid and count them. This plant has flowers about 1.5-2" across. It looks like it holds its color better than GH. The pictured flower is getting old. I should look at its pollen when another flower opens. There are a few more seedlings from GH that look suspicious that I will count when I get a chance. 'Rise ‘N Shine’ (a tetraploid) has produced diploids for me in the past too (mother of GH). I suspect some parents are more prone to it and it may be related to their “genetic load”. Perhaps the process of producing embryos at a low rate from unfertilized eggs is common, but depending on how many poorer forms of genes the unfertilized egg gets, it may impact the new embryo with some being so weak they abort along the way. We’ll see if this seedling has some fertility and could be useful for breeding at the diploid level. It may be nice to cross with diploid species. This seedling seems mildew resistant like GH. Hopefully it inherited the likely two black spot resistance genes GH has as well.


David,
Very cool. I am reminded of what Percy Wright wrote:
American Rose Annual 49:173-175 (1964)
Chromosome Number in Roses
Percy H. WrightThe rules of heredity as worked out by Gregor Mendel were for diploid species. The picture is somewhat simpler than in the case of tetraploids. When we are working with simple rules, we are more likely to reach our goals quickly. In addition, it should be easier to attain the maximum of a given quality, say yellow color in the petals, if there is no second chromosome which fails to carry the same gene for color in the second set of chromosomes of tetraploids.
Why does someone not undertake to put pollen of ‘Persian Yellow’ (> R. foetida persiana> ) on one of the nearest-to-yellow teas? The hybrid so produced should make a proportion of haploid pollen cells. These, fertilizing the teas again, should create a new race of roses in which it should be somewhat easier to intensify the yellow color than in our existing hybrid teas. Also, it should be easier to avoid inheriting the gene or genes for susceptibility to blackspot which come down from ‘Persian Yellow.’
Wright: Chromosome number in Roses (1964)
Part of the advantage of working with diploids rather than polyploids involves “crossover” frequency.
The Evolution of Genetic Systems (1958) pp 151-155
C. D. Darlington
“… the frequency of chiasmata per chromosome is always reduced in a tetraploid owing to the larger nucleus and the slower pairing…”
http://bulbnrose.x10.mx/Heredity/Darlington/DarlingtonSterility1958.html
Very cool indeed! I hope it is a good one and is fertile. So it carries half the genetic information of GH and with no duplications that would occur with a self?
I wish I could raise a hundred of these from say, Carefree Beauty. The very best ones would be very useful for creating terminal triploids and extremely fun for breeding back to diploids.
Mr. Radner sure releases a lot of triploids. I wonder if he has a few very nice dihaploids in his arsenal…
Baxter
I haven’t tried it, but here’s a possibility. Irradiated pollen sprouts, but cannot fertilize.
Agronomie, EDP Sciences, 1994, 14 (3), pp.169-175.
Dihaploid plants of roses (Rosa x hybrida, cv ’Sonia’) obtained by parthenogenesis induced using irradiated pollen and in vitro culture of immature seeds
J Meynet, R Barrade, A Duclos, R Siadous
Hi Karl and Baxter!
That is a great idea. They used tissue culture and embryo rescue, but if the embryo is somewhat viable, hopefully it can develop to maturity on the mother and could result in a higher yield of dihaploids than just looking for them to form normally. Maybe crossing Carefree Beauty with one time blooming species and looking for rare rebloomers as young seedlings would help to find some. There was a seedling years ago out of CB that I suspect was a dihaploid, but I lost it after a couple years.
I’ll post a picture of pollen from this rose when it blooms again. Often dihaploids (in potatoes and it seems in roses too), if fertile, seem to produce normal pollen (in this case 1x), but also tend to produce a good number of unreduced or 2n pollen grains (2x) as well and maybe can serve as a good bridge in going up in ploidy again if that is of interest to people.
I wonder if r. Bracteata pollen could be used to induce these, or is it still too genetically close, producing inviable triploid embryos?
Think I’ll try some on Rise n Shine next year to find out.
Hi Baxter!!
I think that is a great idea. People have used corn pollen on wheat to induce this. It sounds like fertilization occurs, but then the corn chromosomes are preferentially eliminated. They have done this in oats too, but with oats there are often some chromosomes that remain, which has opened up some very interesting research.
David,
Similar, but somewhat more confusing results have been observed when potatoes (Solanum tuberosum) are pollinated by S. phureja. Sometimes the pollen stimulates parthenogenic development. Other times, the pollen nucleus enters the ovum, then the paternal chromosomes are eliminated … but not without leaving some “foot prints” on the resulting dihaploids.
The difference in behavior is influenced by the selected parents. Some potato cultivars accept more Phureja influence, and some Phureja clones are more determined to leave their mark.
Genome. 2004 Aug;47(4):633-8.
Assessment of genetic variability of haploids extracted from tetraploid (2n = 4x = 48) Solanum tuberosum.
Ercolano MR, Carputo D, Li J, Monti L, Barone A, Frusciante L.
“The characterization for resistance to Erwinia carotovora subsp. carotovora and potato virus X (PVX) provided evidence that haploids may express traits that are lacking in the tetraploids they come from, which can be useful for both genetic studies and breeding purposes. It is noteworthy that genotypes combining resistance to both diseases and good pollen stainability were identified. Other possible breeding implications owing to the presence of S. phureja genome in the haploids analyzed are discussed.”
It could be interesting to use Rosa bracteata pollen in an effort to stimulate haploids, especially if that pollen could leave some useful “foot prints” to mark its involvement.
Come to think of it, Tantau’s cross of ‘Baby Chateau’ x Rosa roxburghii did not result in any “normal” hybrids. ‘Floradora’, ‘Käthe Duvigneau’, and ‘Cinnabar’ show no obvious external signs of the pollen parent, except that they are reportedly more resistant to fungal infections than the seed parent. Wulff (1954) stated that, “Only anatomical studies revealed a certain similarity and relationship to the latter species.” I have not yet found a published report on these studies.
Karl
Karl and David,
Maybe the ultimate pollen parent that induces significantly more dihaploids is out there waiting for discovery or development. Maybe more than one exists depending on the genetic makeup of the target maternal tetraploid. Perhaps carefully thought out species crosses could work, as could existing ‘sterile’ species crosses, and the distant members of Rosa discussed above might work, or crosses of them, and even within the much more distantly related non-Rosa members of Rosoideae we might find a few worth investigating. Your educated guesses would be better than mine.
Finding out would require a lot of pollinations with very poor success rates, requiring patience, accurate data collection of experimental conditions and variables, followed by very close attention to viable results, with statistical analyses at the conclusion of all phases. Given the huge number of variables involved perhaps initially it would be best to select only the set of ideas above deemed best, and it could be narrowed further to focus on tetraploid pollen receivers with at least some reported dihaploid production levels.
That sounds like it could be a great grad student or a bright senior level undergrad project, depending on how wide we cast the initial test subject net and whether or not we vary environmental factors.
Dr Z.
If you think some variation of my ideas above might work in your group, and you have a student or two in need of a task that you think would be good fits, I’d like to visit with you regarding some kind of funding. As a former grad student I know what it’s like trying to find funding for a project that would both interest me and give me hope of a possible of improvement on my subject’s collective knowledge. Partial funding, with access only to allowed research results and perhaps a few own-rooted examples of the best resulting cultivars to play with would be best for me, especially if we could wrangle up a few more like-minded participants to pitch in. If the project needs to be more advanced and would require a high level of funding, with an opportunity to participate in future revenues, I would still listen. It would not be my first choice. If I passed on the later case, I would make an effort to find another interested party if you desired.
And if I just broke any Forum rules, or offended anyone in this post, I apologize in advance. Once, again I’m in this for the pure joy of learning and the hope of being able to contribute to the greater good of my favorite Genus in some fashion. And who knows, that greater good may arise by my “dumb ideas” inspiring others into different directions.
Baxter
RBaxterx@gmail.com
There is another matter to consider. Some “blood lines” may be more predisposed to producing dihaploid seedlings than other lines. As David mentioned in the first post on this thread, both ‘Gaye Hammond’ and its seed parent, 'Rise ‘N Shine’, have produced dihaploid offspring.
On the other hand, some lines seem more inclined to “fill in the blanks” by duplicating chromosomes to complete the complement. In the case I mentioned of Tantau’s use of R. roxburghii pollen on ‘Baby Chateau’, the three seedlings were tetraploid like the seed parent. The pollen parent is diploid. And a couple of generations back in the lineage of ‘Baby Chateau’ we find ‘Robin Hood’ (diploid) x ‘J.C. Thornton’ (tetraploid) giving the tetraploid ‘Eva’.
The same difference of behavior has been observed in strawberries. In one case, diploid x octoploid gave a diploid seedling. In another, diploid x octoploid gave at least one octoploid (from a diploid seed parent!). Most seedlings in both crosses were “normal” pentaploids.
Karl
Here are some pictures of pollen from the dihaploid. Some pollen is about 32 microns, typical for pollen that has one set of chromosomes. Some pollen is about 38 microns, typical for unreduced pollen that has 2 sets of chromosomes. There are even very few grains that are about 46 microns that would be typical for pollen with 4 sets of chromosomes. When sex cells start on the male side, there is a cell called the microspore mother cell. It is a typical diploid cell. Each chormosome gets duplicated as if it is getting ready for a normal cell division. In the end for the normal process, things result in four pollen grains each with one set of chromosomes. The duplicated chromosomes of the two sets of chromosomes in the starting diploid cell break apart for 4 sets of chromosomes and somehow everything gets packaged into one pollen grain. There is a lot of aborted pollen grains here too. It looks like for it to be successful as a male one would need to gather a lot of pollen and put it on heavily and then could expect a mix of ploidy levels in the offspring.
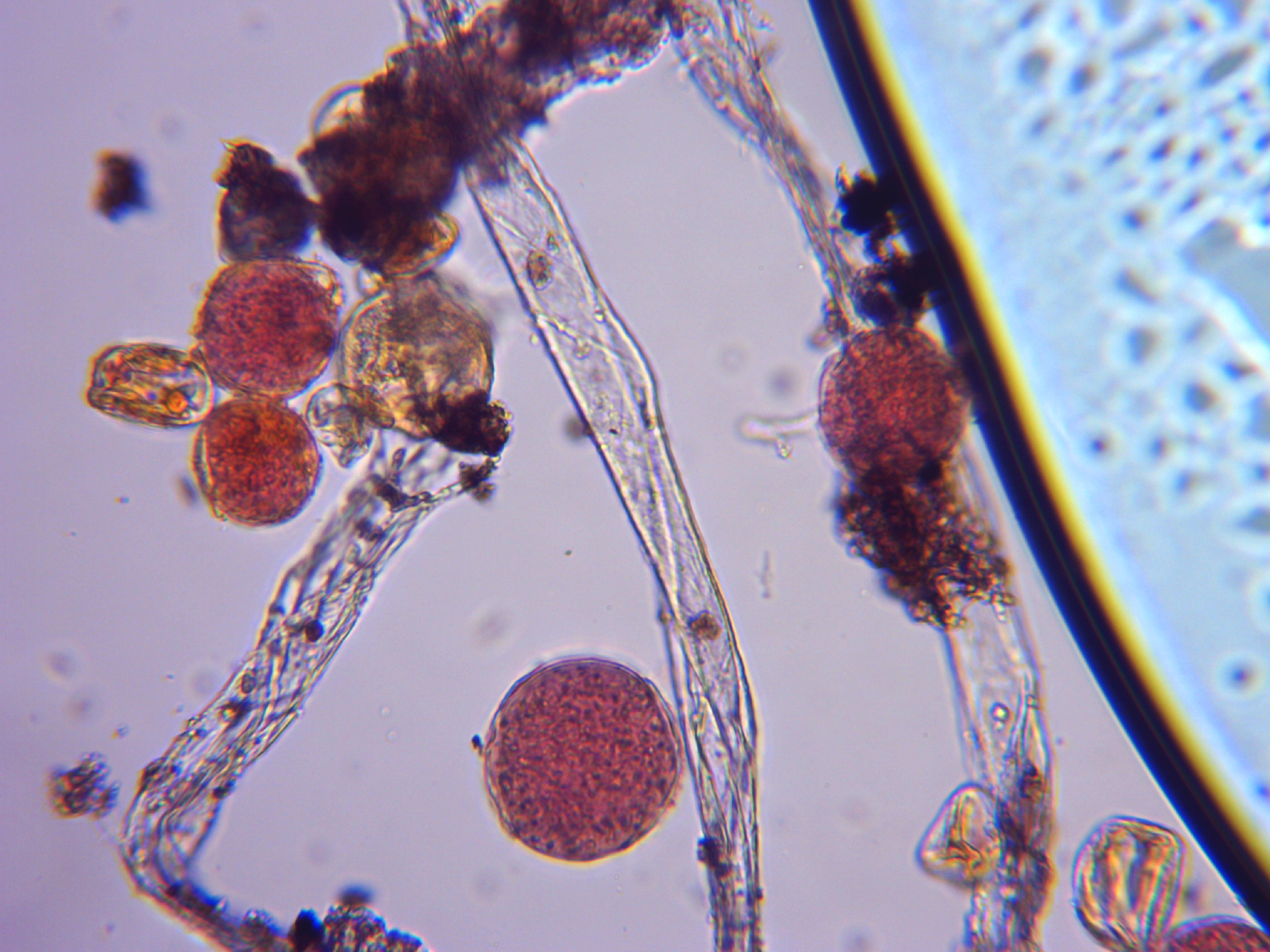
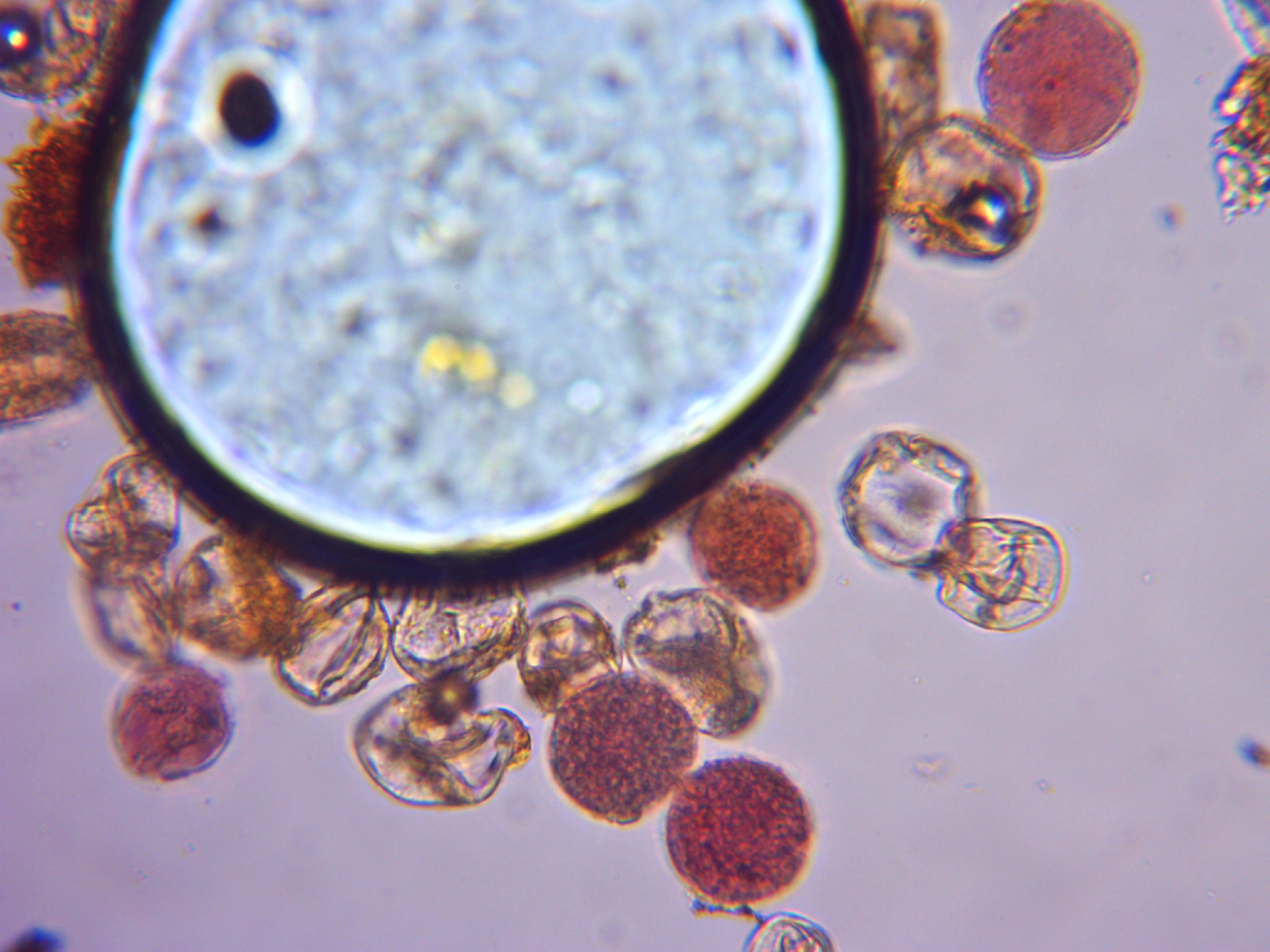
Sorry to all if this is “off topic”.
David Z and or others, is there any registar of roses that have had their ploidy levels done ?
Hi David,
That’s a great question. I don’t know of any really good registry. Peter Harris and others do a great job listing ploidy on helpmefind as they come across references. I share information with Peter when I count a cultivar to try to have that information available for others. Ellen at Texas A&M working with Dr. Byrne has been doing some counts lately for her MS research which is great. Otherwise I don’t know of anyone actively doing a lot of counts right now. I have several Kordes rose samples in the refrigerator that are ready to look at when I have some time, but with the semester in full swing it is difficult now. Periodically people publish papers and ploidy on the roses they are working with if it makes sense for them to count them for their research, which is nice. Years ago Modern Roses had ploidy information for cultivars. They removed that information on cultivars, but left it on species. I prodded why and encouraged to leave it in for cultivars. I don’t understand the editor’s reasoning and maybe they didn’t understand the value of that information for breeders like us.
David,
I haven’t tried this method. Maybe someone else would be interested in experimenting. I think it would be particularly useful for plants with very low pollen viability, because it would allow more good pollen to be piled on the stigma.
Memoirs, Horticultural Society of New York (1902)
Notes on California Plant Breeding
W. H. MORSE
“Pollen grains vary in size and vitality, though they may have been grown in the same stamen. In fact, I am selecting my pollen grains. Method used: A piece of unglazed paper is used, shaking the ripe pollen onto it and curving the paper, and at the same time elevating one end so that the pollen runs down onto a plate. On looking at the pollen with a lens we find that a certain amount of inferior grains are left on the paper, and by repeating the operation only the heaviest grains reach the plate.”
Thank you David Z.